Background:
Since the introduction of novel anti-myeloma agents, multiple studies have shown disparate survival outcomes among African American (AA) patients compared to whites with multiple myeloma (MM). Limited access to novel therapies and autologous hematopoietic stem cell transplant (auto-HCT) has been considered partly responsible for the lower survival outcomes in AA, but other disease-related features may also contribute to this disparity. We hypothesize that patients receiving ASCT would have equal healthcare access, which may nullify the impact of disparate access to novel drugs and healthcare facilities. To test this hypothesis, we compared survival outcomes of AA and whites with MM who underwent upfront ASCT at our center through propensity score matching analysis.
Methods and patients:
A total of 705 MM patients, including AA and whites, who underwent auto-HCT at our institution from 2007 to 2015. By using 1:1 propensity-matching, we identified 251 patients, 125 AA and 126 whites. Clinical response, relapse, and progression were defined by the International Myeloma Working Group criteria.
Results:
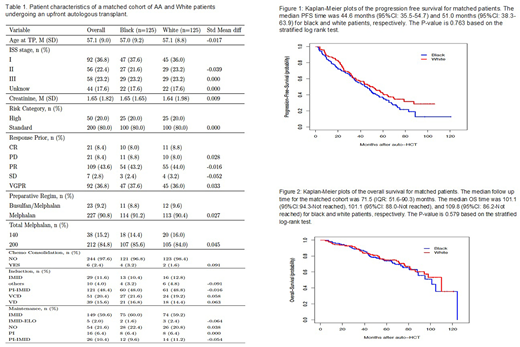
Table 1 includes the baseline characteristics of the matched doublets. Patients in the two groups were well matched for age at auto-HCT, ISS stage, serum creatinine, induction, response to induction, consolidation, preparative regimen, and maintenance therapy. The median follow-up for the matched cohort was 71.5 (interquartile range: 51.6-90.3) months. The overall response rate (CR+VGPR+PR) after auto-HCT was 95.2% (119/125 patients)) and 98.4% (123/125 patients) in the AA and the white group, respectively (p = 0.289). Thirty-four (27.2%) patients achieved a CR in each group. Sixty (48.0%) and 62 (49.6%) patients achieved a VGPR in the AA and the white group, respectively. The median PFS for the AA and the white group was 44.6 (95%CI: 35.5 - 54.7) and 51.0 (95%CI: 38.3 - 63.9) months, respectively (p = 0.763, stratified log-rank test). The 4-year PFS in the AA and the white group was 48% (95%CI: 39.3 - 57.8) and 51.2% (95%CI: 43.0 - 61.0), respectively (Fig. 1). The 4-year OS in the AA and the white group was 78.5% (95%CI: 71.5 - 86.2) and 80.9% (95%CI: 74.1 - 88.2), respectively (Fig. 2).
Conclusions:
In this propensity score matching analysis of MM patients who underwent an auto-HCT at our institution, we showed that AA patients had similar response rates, PFS, and OS as white patients
Bashir:KITE: Other: Advisory Board; Amgen: Other: Advisory Board; Purdue: Other: Advisory Board; Celgene: Research Funding; StemLine: Research Funding; Takeda: Other: Advisory Board, Research Funding; Acrotech: Research Funding. Popat:Bayer: Research Funding; Novartis: Research Funding. Hosing:NKARTA Inc.: Consultancy. Nieto:Secura Bio: Other: Grant Support; Affimed: Consultancy, Other: Grant Support; Astra Zeneca: Other: Grant Support; Novartis: Other: Grant Support. Kebriaei:Amgen: Other: Research Support; Ziopharm: Other: Research Support; Novartis: Other: Served on advisory board; Jazz: Consultancy; Kite: Other: Served on advisory board; Pfizer: Other: Served on advisory board. Alousi:Incyte: Honoraria, Research Funding; Therakos: Research Funding; Alexion: Honoraria. Mehta:Kadmon: Research Funding; Incyte: Research Funding; CSL Behring: Research Funding. Khouri:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding. Thomas:Genentech: Research Funding; BMS: Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Other: Advisory Boards; X4 Pharma: Research Funding; Xencor: Research Funding. Lee:Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Regeneron: Research Funding; Daiichi Sankyo: Research Funding; Genentech: Consultancy; Amgen: Consultancy, Research Funding. Patel:Janssen: Consultancy, Research Funding; Oncopeptides: Consultancy; Nektar: Consultancy, Research Funding; Precision Biosciences: Research Funding; Takeda: Consultancy, Research Funding; Cellectis: Research Funding; Celgene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Poseida: Research Funding. Orlowski:Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties; Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; STATinMED Research: Consultancy. Champlin:Actinium: Consultancy; Johnson and Johnson: Consultancy; Cytonus: Consultancy; Omeros: Consultancy; Genzyme: Speakers Bureau; DKMS America: Membership on an entity's Board of Directors or advisory committees; Takeda: Patents & Royalties. Qazilbash:Bioclinica: Consultancy; Angiocrine: Research Funding; Amgen: Research Funding; Bioline: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.